 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| FGB-H5259 | Human | Human FGF R3 (IIIb) / FGF R3B / CD333 Protein, Fc Tag (MALS verified) |  |

|
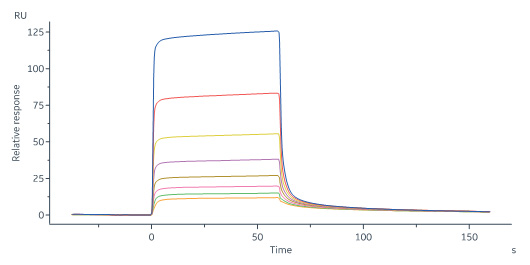
Human FGF R3 (IIIb), Fc Tag (Cat. No. FGB-H5259) immobilized on CM5 Chip can bind Human FGF acidic, Tag Free (Cat. No. AFF-H4116) with an affinity constant of 5.91 μM as determined in a SPR assay (Biacore 8K) (Routinely tested).
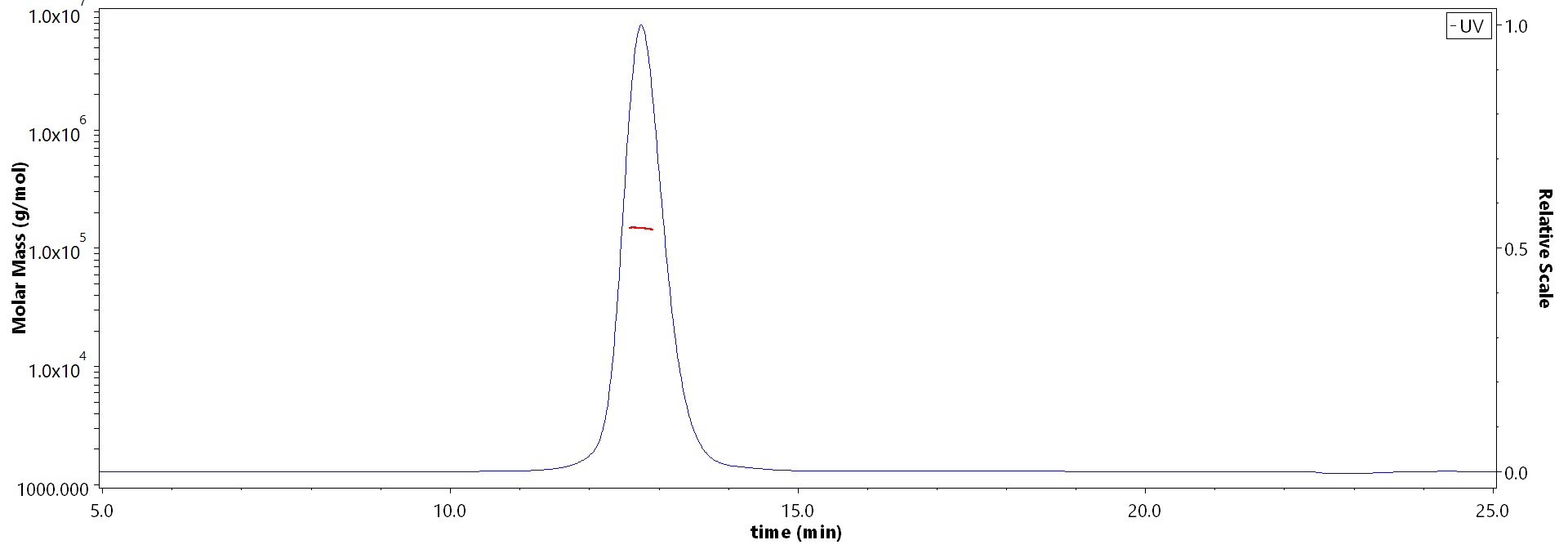
The purity of Human FGF R3 (IIIb), Fc Tag (Cat. No. FGB-H5259) is more than 90% and the molecular weight of this protein is around 132-162 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Carcinoma, Non-Small-Cell Lung | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Sarcoma, Alveolar Soft Part; Bile Duct Diseases; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Hepatic Insufficiency; Bone Neoplasms; Urologic Neoplasms; Fallopian Tube Neoplasms; Thyroid Neoplasms; Endometrial Neoplasms; Medullary thyroid cancer (MTC); Gallbladder Neoplasms; Glioma; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Osteoma; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Ovarian Epithelial; Leiomyosarcoma; Solid tumours; Drug-Related Side Effects and Adverse Reactions; Biliary Tract Neoplasms; Head and Neck Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Sarcoma, Synovial; Neuroendocrine Tumors; Lung Diseases, Interstitial; Liver Diseases; Sarcoma; Nasopharyngeal Carcinoma | Details |
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | United States | Idiopathic Pulmonary Fibrosis | Boehringer Ingelheim Gmbh | 2014-10-15 | Lung Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Adenocarcinoma, Clear Cell; systemic sclerosis-associated interstitial lung disease; Colorectal Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Hepatic Insufficiency; Astrocytoma; Genital Neoplasms, Female; Silicosis; Sarcoma; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Appendiceal Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Endometrioid; Scleroderma, Systemic; Telangiectasia, Hereditary Hemorrhagic; Solid tumours; Rejection of lung transplantation; Carcinoma, Renal Cell; Radiation Pneumonitis; Esophageal Neoplasms; Carcinoid Tumor; Endometrial Stromal Tumors; Idiopathic Pulmonary Fibrosis; Neoplasms; Ovarian Neoplasms; Glioblastoma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pulmonary Fibrosis; Lung Diseases, Interstitial; Oligodendroglioma; Multiple Myeloma; Mesothelioma; Asbestosis; Neuroendocrine Tumors | Details |
| Futibatinib | TAS-120 | Approved | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | LYTGOBI | United States | Cholangiocarcinoma | Taiho Oncology Inc | 2022-09-30 | Biliary Tract Neoplasms; Solid tumours; Bone Marrow Neoplasms; Carcinoma; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Central Nervous System Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Sarcoma; Endometrial Neoplasms; Lymphoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Pemigatinib | INCB-054828; INCB-54828; IBI-375 | Approved | Incyte Corp | Pemazyre, 伯坦, 达伯坦 | United States | Cholangiocarcinoma | Incyte Corp | 2020-04-17 | Multiple Myeloma; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Glioma; Lung Neoplasms; Lymphoma; Colitis, Ulcerative; Bile Duct Neoplasms; Colorectal Neoplasms; Urologic Neoplasms; Cholangiocarcinoma; Translocation, Genetic; Breast Neoplasms; Bone Marrow Neoplasms; Urinary Bladder Neoplasms; Glioblastoma; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Myeloproliferative Disorders; Carcinoma; Carcinoma, Renal Cell; Stomach Neoplasms; Biliary Tract Neoplasms; Solid tumours | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | United States | Carcinoma, Renal Cell; Sarcoma | Novartis Pharma Ag | 2009-10-19 | Uterine Cervical Diseases; Fallopian Tube Neoplasms; Lung Neoplasms; Uterine Neoplasms; Choriocarcinoma; Lymphoma; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Mucoepidermoid; Gliosarcoma; Genital Neoplasms, Female; Leukemia, Myeloid, Acute; Peritoneal Neoplasms; Brain Neoplasms; Urethral Neoplasms; Medullary thyroid cancer (MTC); Chondrosarcoma, Extraskeletal Myxoid; Prostatic Neoplasms; Osteosarcoma; Neuroblastoma; Sarcoma; Breast Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Neoplasm Metastasis; von Hippel-Lindau Disease; Thyroid Cancer, Papillary; Gastrointestinal Neoplasms; Paraganglioma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Melanoma; Gastrinoma; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Small Cell; Carcinoma, Embryonal; Germinoma; Glioma; Carcinoma, Neuroendocrine; Epistaxis; Carcinoma, Ovarian Epithelial; Neoplasms; Squamous Cell Carcinoma of Head a | Details |
| Erdafitinib | G-024; JNJ-493; JNJ-42756493; TAR-210; 890E37NHMV | Approved | Astex Pharmaceuticals Inc | Balversa | United States | Carcinoma, Transitional Cell | Janssen Biotech Inc | 2019-04-12 | Carcinoma, Squamous Cell; Breast Neoplasms; Neuroblastoma; Sarcoma; Prostatic Neoplasms; Bile Duct Neoplasms; Urologic Neoplasms; Histiocytic Sarcoma; Hepatic Insufficiency; Lymphoma; Lymphoma, Non-Hodgkin; Histiocytosis, Langerhans-Cell; Carcinoma, Neuroendocrine; Neoplasms, Neuroepithelial; Glioma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Xanthogranuloma, Juvenile; Neuroectodermal Tumors, Primitive, Peripheral; Neoplasms, Germ Cell and Embryonal; Carcinoma, Transitional Cell; Medulloblastoma; Rhabdomyosarcoma; Hematologic Neoplasms; Ependymoma; Bone metastases; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Rhabdoid Tumor; Hepatoblastoma; Solid tumours; Neoplasms; Wilms Tumor; Glioblastoma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Multiple Myeloma; Prostatic Neoplasms, Castration-Resistant; Sarcoma, Ewing; Osteosarcoma | Details |
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Carcinoma, Non-Small-Cell Lung | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Sarcoma, Alveolar Soft Part; Bile Duct Diseases; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Hepatic Insufficiency; Bone Neoplasms; Urologic Neoplasms; Fallopian Tube Neoplasms; Thyroid Neoplasms; Endometrial Neoplasms; Medullary thyroid cancer (MTC); Gallbladder Neoplasms; Glioma; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Osteoma; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Ovarian Epithelial; Leiomyosarcoma; Solid tumours; Drug-Related Side Effects and Adverse Reactions; Biliary Tract Neoplasms; Head and Neck Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Sarcoma, Synovial; Neuroendocrine Tumors; Lung Diseases, Interstitial; Liver Diseases; Sarcoma; Nasopharyngeal Carcinoma | Details |
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | United States | Idiopathic Pulmonary Fibrosis | Boehringer Ingelheim Gmbh | 2014-10-15 | Lung Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Adenocarcinoma, Clear Cell; systemic sclerosis-associated interstitial lung disease; Colorectal Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Hepatic Insufficiency; Astrocytoma; Genital Neoplasms, Female; Silicosis; Sarcoma; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Appendiceal Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Endometrioid; Scleroderma, Systemic; Telangiectasia, Hereditary Hemorrhagic; Solid tumours; Rejection of lung transplantation; Carcinoma, Renal Cell; Radiation Pneumonitis; Esophageal Neoplasms; Carcinoid Tumor; Endometrial Stromal Tumors; Idiopathic Pulmonary Fibrosis; Neoplasms; Ovarian Neoplasms; Glioblastoma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pulmonary Fibrosis; Lung Diseases, Interstitial; Oligodendroglioma; Multiple Myeloma; Mesothelioma; Asbestosis; Neuroendocrine Tumors | Details |
| Futibatinib | TAS-120 | Approved | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | LYTGOBI | United States | Cholangiocarcinoma | Taiho Oncology Inc | 2022-09-30 | Biliary Tract Neoplasms; Solid tumours; Bone Marrow Neoplasms; Carcinoma; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Central Nervous System Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Sarcoma; Endometrial Neoplasms; Lymphoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Pemigatinib | INCB-054828; INCB-54828; IBI-375 | Approved | Incyte Corp | Pemazyre, 伯坦, 达伯坦 | United States | Cholangiocarcinoma | Incyte Corp | 2020-04-17 | Multiple Myeloma; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Glioma; Lung Neoplasms; Lymphoma; Colitis, Ulcerative; Bile Duct Neoplasms; Colorectal Neoplasms; Urologic Neoplasms; Cholangiocarcinoma; Translocation, Genetic; Breast Neoplasms; Bone Marrow Neoplasms; Urinary Bladder Neoplasms; Glioblastoma; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Myeloproliferative Disorders; Carcinoma; Carcinoma, Renal Cell; Stomach Neoplasms; Biliary Tract Neoplasms; Solid tumours | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | United States | Carcinoma, Renal Cell; Sarcoma | Novartis Pharma Ag | 2009-10-19 | Uterine Cervical Diseases; Fallopian Tube Neoplasms; Lung Neoplasms; Uterine Neoplasms; Choriocarcinoma; Lymphoma; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Mucoepidermoid; Gliosarcoma; Genital Neoplasms, Female; Leukemia, Myeloid, Acute; Peritoneal Neoplasms; Brain Neoplasms; Urethral Neoplasms; Medullary thyroid cancer (MTC); Chondrosarcoma, Extraskeletal Myxoid; Prostatic Neoplasms; Osteosarcoma; Neuroblastoma; Sarcoma; Breast Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Neoplasm Metastasis; von Hippel-Lindau Disease; Thyroid Cancer, Papillary; Gastrointestinal Neoplasms; Paraganglioma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Melanoma; Gastrinoma; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Small Cell; Carcinoma, Embryonal; Germinoma; Glioma; Carcinoma, Neuroendocrine; Epistaxis; Carcinoma, Ovarian Epithelial; Neoplasms; Squamous Cell Carcinoma of Head a | Details |
| Erdafitinib | G-024; JNJ-493; JNJ-42756493; TAR-210; 890E37NHMV | Approved | Astex Pharmaceuticals Inc | Balversa | United States | Carcinoma, Transitional Cell | Janssen Biotech Inc | 2019-04-12 | Carcinoma, Squamous Cell; Breast Neoplasms; Neuroblastoma; Sarcoma; Prostatic Neoplasms; Bile Duct Neoplasms; Urologic Neoplasms; Histiocytic Sarcoma; Hepatic Insufficiency; Lymphoma; Lymphoma, Non-Hodgkin; Histiocytosis, Langerhans-Cell; Carcinoma, Neuroendocrine; Neoplasms, Neuroepithelial; Glioma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Xanthogranuloma, Juvenile; Neuroectodermal Tumors, Primitive, Peripheral; Neoplasms, Germ Cell and Embryonal; Carcinoma, Transitional Cell; Medulloblastoma; Rhabdomyosarcoma; Hematologic Neoplasms; Ependymoma; Bone metastases; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Rhabdoid Tumor; Hepatoblastoma; Solid tumours; Neoplasms; Wilms Tumor; Glioblastoma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Multiple Myeloma; Prostatic Neoplasms, Castration-Resistant; Sarcoma, Ewing; Osteosarcoma | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Lucitanib | S-80881; AL-3810; CO-3810; E-3810; S-80881-2 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Rogaratinib | BAY-1163877 | Phase 3 Clinical | Bayer AG | Solid tumours; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| Fexagratinib | AZD4547; AZD-4547; ABSK-091; ABSK091 | Phase 3 Clinical | Astrazeneca Plc | Uterine Neoplasms; Multiple Myeloma; Breast Neoplasms; Prostatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Squamous Cell; Glioma; Urinary Bladder Neoplasms; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Foodborne Diseases; Adenocarcinoma; Uterine Cervical Neoplasms; Rectal Neoplasms; Solid tumours; Hematologic Neoplasms; Ovarian Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Gastrointestinal Diseases; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Liver Neoplasms; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Neoplasms; Colonic Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Skin Neoplasms | Details |
| Infigratinib | BGJ-398; NVP-BGJ398 | Phase 3 Clinical | Novartis Pharma Ag | Papillomavirus Infections; Adenocarcinoma; Uterine Cervical Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Squamous Cell; Glioma; Oropharyngeal Neoplasms; Gastrointestinal Stromal Tumors; Breast Neoplasms; Cholangiocarcinoma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Glioblastoma; Biliary Tract Neoplasms; Neoplasms; Achondroplasia; Nasopharyngeal Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Hematologic Neoplasms; Solid tumours | Details |
| Efruxifermin | AKR-001; AMG-876; EFX; Fc-FGF21(RGE) | Phase 3 Clinical | Amgen Inc | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus | Details |
| SC-0011 | SC-0011 | Phase 3 Clinical | Shijiazhuang Zhikang Hongren New Drug Development Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Zoligratinib | CH-5183284; FF-284; Debio-1347 | Phase 2 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Breast Neoplasms | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms | Details |
| Derazantinib | ARQ-087.2HCl; AQ-14741087; ARQ-087; BAL-087 | Phase 2 Clinical | Arqule Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma; Urogenital Neoplasms; Carcinoma, Hepatocellular | Details |
| HH185 | 3D-185; 3-D185; HH-185; 3D185 | Phase 2 Clinical | ShangHai HaiHe Biopharma Co Ltd, Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences, Shanghai Medicilon Inc | Solid tumours; Cholangiocarcinoma | Details |
| TYRA-300 | TYRA300 | Phase 2 Clinical | Tyra Biosciences Inc | Solid tumours; Carcinoma; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Urologic Neoplasms | Details |
| SAR-442501 | SAR-442501 | Phase 2 Clinical | Sanofi | Osteochondrodysplasias; Achondroplasia | Details |
| HMPL-453 | HMPL-453 | Phase 2 Clinical | Hutchison Medipharma Ltd | Solid tumours; Biliary Tract Neoplasms; Neoplasms, Mesothelial; Mesothelioma; Cholangiocarcinoma | Details |
| Tasurgratinib | E-7090 | Phase 2 Clinical | Eisai Co Ltd | Biliary Tract Neoplasms; Solid tumours; Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Hepatic Insufficiency | Details |
| Sprifermin | rhFGF-18; trFGF-18; FGF-18; zFGF-5; Zfgf5; AS-902330 | Phase 2 Clinical | Zymogenetics Inc | Cartilage Diseases; Knee Injuries; Osteoarthritis, Knee | Details |
| Gunagratinib | ICP-192 | Phase 2 Clinical | Beijing Tiancheng Pharmaceutical Technology Co Ltd | Solid tumours; Biliary Tract Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Cholangiocarcinoma; Lung Neoplasms | Details |
| Recifercept | PF-07256472; TA-46 | Phase 1 Clinical | Pfizer Inc | Achondroplasia | Details |
| BPI-17509 | BPI-17509 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Solid tumours | Details |
| TYRA-200 | TYRA200; TYRA-200 | Phase 1 Clinical | Tyra Biosciences Inc | Biliary Tract Neoplasms; Solid tumours; Cholangiocarcinoma; Bile Duct Neoplasms | Details |
| Resigratinib | KIN-3248; KIN-003 | Phase 1 Clinical | Kinnate Biopharma Inc | Solid tumours; Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma | Details |
| TYRA-300-B01 | TYRA-300-B01 | Phase 1 Clinical | Tyra Biosciences Inc | Details | |
| LOXO-435 | LOXO-435 | Phase 1 Clinical | Eli Lilly And Company | Solid tumours; Neoplasms; Urinary Bladder Neoplasms; Ureteral Neoplasms; Neoplasm Metastasis | Details |
| TT-00434 | TT-00434 | Phase 1 Clinical | TransThera Sciences (Nanjing) Inc | Solid tumours; Urinary Bladder Neoplasms | Details |
| ABSK-121 | ABSK121-NX; ABSK121 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours | Details |
| ABSK-061 | ABSK061 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours | Details |
| LY-2874455 | LY-2874455 | Phase 1 Clinical | Eli Lilly And Company | Neoplasms; Leukemia, Myeloid, Acute | Details |
| CPL-304110 | CPL-304110; CPL-304-110 | Phase 1 Clinical | Celon Pharma Sa | Stomach Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Cholangiocarcinoma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung | Details |
| Lucitanib | S-80881; AL-3810; CO-3810; E-3810; S-80881-2 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Rogaratinib | BAY-1163877 | Phase 3 Clinical | Bayer AG | Solid tumours; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| Fexagratinib | AZD4547; AZD-4547; ABSK-091; ABSK091 | Phase 3 Clinical | Astrazeneca Plc | Uterine Neoplasms; Multiple Myeloma; Breast Neoplasms; Prostatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Squamous Cell; Glioma; Urinary Bladder Neoplasms; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Foodborne Diseases; Adenocarcinoma; Uterine Cervical Neoplasms; Rectal Neoplasms; Solid tumours; Hematologic Neoplasms; Ovarian Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Gastrointestinal Diseases; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Liver Neoplasms; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Neoplasms; Colonic Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Skin Neoplasms | Details |
| Infigratinib | BGJ-398; NVP-BGJ398 | Phase 3 Clinical | Novartis Pharma Ag | Papillomavirus Infections; Adenocarcinoma; Uterine Cervical Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Squamous Cell; Glioma; Oropharyngeal Neoplasms; Gastrointestinal Stromal Tumors; Breast Neoplasms; Cholangiocarcinoma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Glioblastoma; Biliary Tract Neoplasms; Neoplasms; Achondroplasia; Nasopharyngeal Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Hematologic Neoplasms; Solid tumours | Details |
| Efruxifermin | AKR-001; AMG-876; EFX; Fc-FGF21(RGE) | Phase 3 Clinical | Amgen Inc | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus | Details |
| SC-0011 | SC-0011 | Phase 3 Clinical | Shijiazhuang Zhikang Hongren New Drug Development Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Zoligratinib | CH-5183284; FF-284; Debio-1347 | Phase 2 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Breast Neoplasms | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms | Details |
| Derazantinib | ARQ-087.2HCl; AQ-14741087; ARQ-087; BAL-087 | Phase 2 Clinical | Arqule Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma; Urogenital Neoplasms; Carcinoma, Hepatocellular | Details |
| HH185 | 3D-185; 3-D185; HH-185; 3D185 | Phase 2 Clinical | ShangHai HaiHe Biopharma Co Ltd, Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences, Shanghai Medicilon Inc | Solid tumours; Cholangiocarcinoma | Details |
| TYRA-300 | TYRA300 | Phase 2 Clinical | Tyra Biosciences Inc | Solid tumours; Carcinoma; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Urologic Neoplasms | Details |
| SAR-442501 | SAR-442501 | Phase 2 Clinical | Sanofi | Osteochondrodysplasias; Achondroplasia | Details |
| HMPL-453 | HMPL-453 | Phase 2 Clinical | Hutchison Medipharma Ltd | Solid tumours; Biliary Tract Neoplasms; Neoplasms, Mesothelial; Mesothelioma; Cholangiocarcinoma | Details |
| Tasurgratinib | E-7090 | Phase 2 Clinical | Eisai Co Ltd | Biliary Tract Neoplasms; Solid tumours; Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Hepatic Insufficiency | Details |
| Sprifermin | rhFGF-18; trFGF-18; FGF-18; zFGF-5; Zfgf5; AS-902330 | Phase 2 Clinical | Zymogenetics Inc | Cartilage Diseases; Knee Injuries; Osteoarthritis, Knee | Details |
| Gunagratinib | ICP-192 | Phase 2 Clinical | Beijing Tiancheng Pharmaceutical Technology Co Ltd | Solid tumours; Biliary Tract Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Cholangiocarcinoma; Lung Neoplasms | Details |
| Recifercept | PF-07256472; TA-46 | Phase 1 Clinical | Pfizer Inc | Achondroplasia | Details |
| BPI-17509 | BPI-17509 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Solid tumours | Details |
| TYRA-200 | TYRA200; TYRA-200 | Phase 1 Clinical | Tyra Biosciences Inc | Biliary Tract Neoplasms; Solid tumours; Cholangiocarcinoma; Bile Duct Neoplasms | Details |
| Resigratinib | KIN-3248; KIN-003 | Phase 1 Clinical | Kinnate Biopharma Inc | Solid tumours; Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma | Details |
| TYRA-300-B01 | TYRA-300-B01 | Phase 1 Clinical | Tyra Biosciences Inc | Details | |
| LOXO-435 | LOXO-435 | Phase 1 Clinical | Eli Lilly And Company | Solid tumours; Neoplasms; Urinary Bladder Neoplasms; Ureteral Neoplasms; Neoplasm Metastasis | Details |
| TT-00434 | TT-00434 | Phase 1 Clinical | TransThera Sciences (Nanjing) Inc | Solid tumours; Urinary Bladder Neoplasms | Details |
| ABSK-121 | ABSK121-NX; ABSK121 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours | Details |
| ABSK-061 | ABSK061 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours | Details |
| LY-2874455 | LY-2874455 | Phase 1 Clinical | Eli Lilly And Company | Neoplasms; Leukemia, Myeloid, Acute | Details |
| CPL-304110 | CPL-304110; CPL-304-110 | Phase 1 Clinical | Celon Pharma Sa | Stomach Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Cholangiocarcinoma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung | Details |
This web search service is supported by Google Inc.
